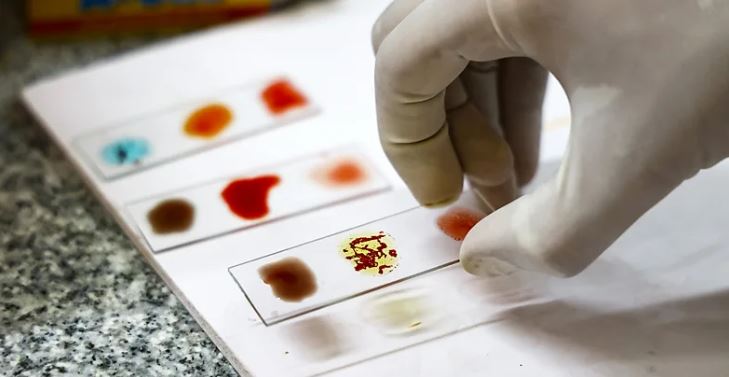
Only one in every six million people have the Rh null blood type. Now researchers are trying to grow it in the laboratory in the hope it could save lives.
Blood transfusions have transformed modern medicine. If we are ever unlucky enough to be injured or need serious surgery, blood that has been donated by others can be life-saving.
But not everyone is able to benefit from this remarkable procedure. People with rare blood types struggle to find donated blood that will match their own.
One of the rarest – the Rh null blood type – is found in just 50 known people in the world. Should they ever be in an accident that needs a transfusion, their chances of getting one are slim. Those with Rh null are instead encouraged to freeze their own blood for long-term storage.
But, despite its rarity, this blood type is also highly prized for other reasons. Within the medical and research community, it is sometimes referred to as "golden blood" due to how it can be used.
It may also help to create universal blood transfusions as scientists search for ways of overcoming the immunity issues that currently restrict how donated blood is used.
The type of blood you have circulating around your body is classified based on the presence or absence of specific markers on the surface of your red blood cells. These markers, known as antigens, consist of proteins or sugars which stick out from the cell surface and can be detected by the body's immune system.
"If you get transfused with donor blood that contains different antigens to your own blood, you'll make antibodies to that blood and attack it," says Ash Toye, professor of cell biology at the University of Bristol. "If you get transfused with that blood again, it can be life threatening."
The two blood group systems that evoke the largest immune response are ABO and Rhesus (Rh). A person with an A blood group has A antigens on the surface of their red blood cells, while someone with a B blood type has B antigens. The AB blood group has both A and B antigens, with the O group has neither. Each group can be either Rh positive or Rh negative.
People with O negative blood are often described as universal donors, as their blood contains neither A, B, or Rh antigens. However, this is an oversimplification.
First, there are currently 47 known blood groups and 366 different antigens, as of October 2024. That means that a person receiving an O negative donation could still have an immune reaction to any of the other antigens present – although some antigens provoke more of an immune response than others.
Secondly, there are over 50 Rh antigens. When people talk about being Rh negative they are referring to the Rh(D) antigen, but their red blood cells still contain other Rh proteins. There is also a huge diversity of Rh antigens across the world, making it challenging to find true donor matches, especially for people from ethnic minority backgrounds in a given country.
People with Rh null blood, however, lack all 50 Rh antigens. While these people cannot receive any other blood type, Rh null blood is compatible with all of the many Rh blood types.
This makes O type Rh null blood extremely valuable, as the majority of people can receive it, including individuals with all variants of ABO. In emergencies where a patient's blood type is not known, O type Rh null blood could be given with a low risk of allergic reaction. For this reason, scientists around the world are looking for ways to replicate this "golden blood".
"Rh [antigens trigger] a large immune response and so if you have none of [them] at all then essentially there's nothing to react to in terms of Rh," says Toye. "If you were type O and Rh null then that's pretty universal. But there are still other blood groups that you still have to consider."
The origin of Rh null blood
Recent research has revealed that Rh null blood is caused by genetic mutations that affect a protein that plays a crucial role in red blood cells, known as Rh associated glycoprotein, or RHAG. These mutations appear to shorten or alter the shape of this protein, causing it to disrupt the expression of other Rh antigens.
In a 2018 study, Toye and colleagues at the University of Bristol recreated Rh null blood in the lab. To do so they took a cell line – a population of cells grown in a laboratory – of immature red blood cells. The team then used the gene editing technique Crispr-Cas9 to delete genes coding for the antigens of the five blood group systems that collectively are responsible for most transfusion incompatibilities. This included the ABO and Rh antigens, as well as other antigens called Kell, Duffy and GPB.
"We worked out if we knocked out five, then that would create an ultra-compatible cell, because it had five of the most problematic blood groups removed," says Toye.
The resulting blood cells would be compatible for all the major common blood groups but also for those with rare types like Rh null and the Bombay phenotype, which is carried by one in every four million people. People with this blood group cannot be given O, A, B or AB blood.
Using gene editing techniques, however, remains controversial and tightly regulated in many parts of the world, which means it could be some time before this ultra-compatible type of blood might become clinically available. It would need to go through many rounds of clinical trials and testing before being approved.
Toye hopes to create banks of rare blood in the laboratory without using gene editing, although the technique could play a role in the future.
In 2021, immunologist Gregory Denomme and colleagues at the Versiti Blood Research Institute, in Milwaukee, US, used Crispr-Cas9 gene editing technology to create customised rare blood types including Rh null from human induced pluripotent stem cells (hiPSC). These stem cells have properties similar to embryonic stem cells and have the potential to become any cell in the human body, given the right conditions.
Other scientists are using another type of stem cells that are already preprogrammed to become blood cells but haven't determined which kind yet. For example scientists at Laval University in Quebec, Canada recently extracted blood stem cells from donors with A positive blood. They then used Crispr-Cas9 technology to delete the genes coding for the A and Rh antigens, producing O Rh null immature red blood cells. Researchers in Barcelona, Spain, also recently took stem cells from a Rh null blood donor, and used Crispr-Cas9 to convert their blood from type A to type O, making it more universal.
Nevertheless, despite these impressive efforts it's important to say that creating artificial lab-grown blood on a scale where people could use it is still a long way off. One difficulty is to get the stem cells to grow into mature red blood cells. In the body, red blood cells are produced from stem cells in the bone marrow, which produces complex signals to guide how they develop. This is difficult to replicate in the laboratory.
"There is the added problem that when creating Rh null or any other null blood type, the growth and maturation of the red blood cells can be perturbed," says Denomme, who is now working as a medical affairs director at Grifols Diagnostic Solutions, a healthcare company which specialises in transfusion medicine. "Producing specific blood group genes might result in the cell membrane falling apart, or a loss of producing red blood cells efficiently in cell culture."
For now, Toye is co-leading the RESTORE trial, the world's first ever clinical trial testing the safety of giving healthy volunteers red blood cells that have been artificially grown in the laboratory from donor blood stem cells. The artificial blood in the trial wasn't gene edited in any way, but it still took 10 years of research to get to the stage where scientists were ready to test it in humans.
"At the moment, taking blood out of somebody's arm is so much more efficient and cost effective, and so we will need blood donors for the foreseeable future," says Toye.
"But for people with rare blood types where there's very few other donors, if we can grow them more blood, that would be really exciting."













![[PHOTOS] How ODM@20 dinner went down](/_next/image?url=https%3A%2F%2Fcdn.radioafrica.digital%2Fimage%2F2025%2F11%2F99d04439-7d94-4ec5-8e18-899441a55b21.jpg&w=3840&q=100)

